In the next few weeks, millions of Americans will be deciding whether to take the SARS-CoV-2 vaccine. My guess is that many people have already made their decision. You may be among the countless Americans who have been eagerly awaiting the availability of a vaccine so you can return to some level of normalcy in your life. Whether you are a proponent or opponent of vaccines, it’s important to make an informed decision so you know what you will be injecting into your body. It’s critical to understand both the effectiveness of the vaccine in preventing SARS-CoV-2 as well as the risks involved with taking the vaccine. After all, “Operation Warp Speed” doesn’t exactly conjure up confidence. It makes one wonder what safety measures were overlooked to push a vaccine through to market in record time? It’s a legitimate question and one worth investigating. In this article, I will address three questions:
1) Is the vaccine effective in preventing SARS-CoV-2?
2) Are there any safety measures that were overlooked during “Operation Warp Speed”?
3) What are the risks associated with the vaccine?
As we consider the effectiveness of a vaccine to prevent a person from becoming infected with SARS-CoV-2, it’s important to keep in mind the survivability rate for a person infected with the virus. To figure this out, we start by looking at total cases and total deaths to see that 1.89% of those who get the virus, die from the virus (see Figure 1).
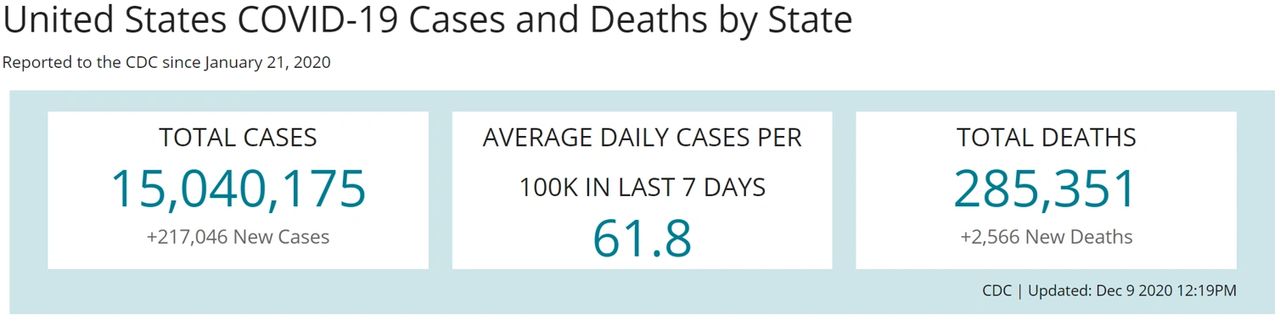
Figure 1. CDC COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
To drill down further, we can look at the Infection Fatality Ratio provided by the CDC (see Figure 2). This table is helpful because you can look at your age range to determine your risk of dying from the virus. For the majority of the population, there
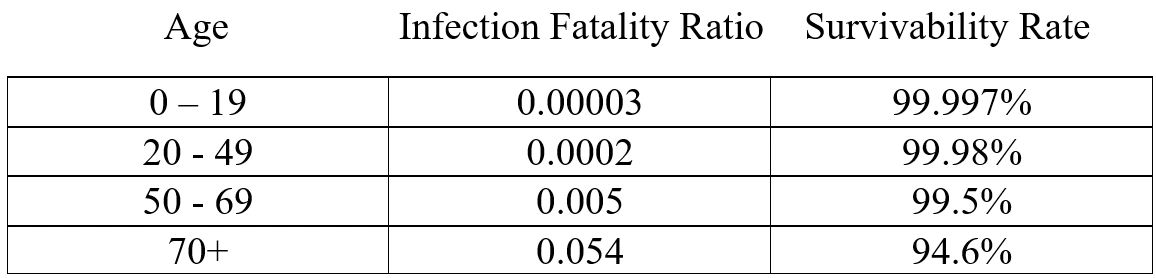
Figure 2: Current Best Estimate Sept 10, 2020 https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html
is less than 1% chance of dying from the virus. Of course, if you have underlying medical conditions it would heighten your risk which is something to keep in mind when considering the vaccine.
Is the vaccine effective in preventing SARS-CoV-2?
Multiple news outlets have reported the vaccines to be 85-95% effective. But effective at what? Well, it turns out the vaccines are not actually effective at preventing SARS-CoV-2. Wait what? Yes, that’s right, they are not actually effective at preventing SARS-CoV-2. Then what are they effective at? Only at minimizing the symptoms of the virus. William Haseltine, the founder of the Division of Biochemical Pharmacology and the Division of Human Retrovirology at Harvard University writes:
“It appears that all the pharmaceutical companies assume that the vaccine will never prevent infection. Their criteria for approval is the difference in symptoms between an infected control group and an infected vaccine group. They do not measure the difference between infection and noninfection as a primary motivation. A greater concern for the millions of older people and those with preexisting conditions is whether these trials test the vaccine’s ability to prevent severe illness and death. Again, we find that severe illness and death are only secondary objectives in these trials. None list the prevention of death and hospitalization as a critically important barrier.”(1) (emphasis mine)
It seems that the criterion for success of the SARS-CoV-2 vaccine was set surprisingly low. “Moderna, Pfizer and AstraZeneca did not require that their vaccine prevent serious disease only that they prevent moderate symptoms which may be as mild as cough, or headache.”(2) A vaccine should be designed to significantly reduce serious illness and death. When most Americans hear that the vaccine is 95% effective, they think if they get the vaccine, they will only have a 5% chance of getting infected with the virus. Sadly, this is not remotely close to accurate. Researchers are also unsure how long the protective measure of the vaccine will last. It’s possible it will only last several months.
So, if a reduction in symptoms is the most the vaccine has to offer, it behooves us to understand the risks associated with it.
Are there any safety measures that were overlooked during “Operation Warp Speed”?
In the race to make a vaccine available to the public, pharmaceutical companies began Phase I/II/III human trials before completing the prerequisite animal studies. This shortcut is a clear break from industry protocol. McGill University’s biomedical ethicist, Jonathan Kimmelman, expressed concern, “Outbreaks and national emergencies often create pressure to suspend rights, standards and/or normal rules of ethical conduct. Often our decision to do so seems unwise in retrospect.”(3) To understand the potential impact of this shortcut, we need to review the animal trials from SARS-CoV vaccine studies.
In 2003, the SARS-CoV epidemic hit the US as well as a number of other countries. Scientists attempted to develop a successful vaccine, but to date, they have been unsuccessful. To be clear, there is NO vaccine for SARS-CoV. Why? Researchers were unable to progress to Phase I human trials because the findings from the animal trials were problematic. At first, the vaccines administered to animals showed great promise because a robust antibody response was found. However, when the animals were later exposed to the virus in the environment, they became gravely ill. The vaccinated animals developed an Antibody Dependent Enhancement (ADE) known as Th2-type immunopathology, a hyper-induced immune response causing inflammation all throughout the body, especially in the lungs. This is referred to as a cytokine storm and is often fatal. These findings alarmed the researchers and they concluded:
“This… provides concern for trials with SARS-CoV vaccines in humans. Clinical trials with SARS coronavirus vaccines have been conducted and reported to induce antibody responses and to be “safe”. However, the evidence for safety is for a short period of observation. The concern arising from the present report is for an immunopathologic reaction occurring among vaccinated individuals on exposure to infectious SARS-CoV, the basis for developing a vaccine for SARS.”(4)
In other words, the very reason to develop a vaccine is to prevent infection and certainly to prevent severe symptoms associated with infection. The animal trials proved that all four types of SARS-CoV vaccines triggered serious infection when the animals were exposed to the virus in the wild. This posed a health risk for human participants involved in the vaccine trials, so the studies were suspended. Similar concerns have been raised with the clinical trials of SARS-CoV-2 vaccine studies.
On December 1, 2020, former Pfizer VP and scientific director, Dr. Michael Yeadon, and German pulmonary specialist, Dr. Wolfgang Wodarg filed an “urgent application with the European Medicine Agency calling for the immediate suspension of all SARS-CoV-2 vaccine studies - particularly the BioNtech/Pfizer study.”(5) They identified the following serious safety concerns:
1) Human trials were started before completion of the animal studies could provide evidence as to whether the vaccine was safe for human trials. The SARS-CoV-2 vaccine can cause some people to develop a non-neutralizing antibody, meaning it fails to protect from infection. If a person becomes infected with the virus after developing non-neutralizing antibodies, it can elicit a more severe reaction (ADE) which is the primary reason the SARS-CoV vaccine trials failed.(6)
2) High cycle thresholds (Ct values) in the PCR tests used to diagnose SARS-CoV-2 in the Pfizer trials “have been widely acknowledged to lead to false positives.” Yeadon and Wodarg assert that positive test results should be verified by Sanger sequencing to confirm the validity of the findings. They argue “if the vaccines are approved without an appropriate and accurate review of efficacy, then any potential acceptance or mandate of these vaccines is likely to be based on inaccurate evidence.”(7)
It’s important to note that the Pfizer and Moderna mRNA vaccines are a technology that has never been licensed for use in humans before. Legally they are considered “experimental vaccines”, but the word “experimental” has been removed from much of the messaging.(8) Furthermore, there has not been adequate time to assess the long-term risks associated with an experimental vaccine of this type. Dr. Carrie Madej points out that an experimental vaccine requires 10 - 15 years of research data to demonstrate its safety before being administered to the public.(9)
What are the risks associated with the vaccine?
1) ADE - a hyperinflammatory response - a cytokine storm. This complication in the immune system can lead to widespread damage of internal organs and death.
2) Anaphylaxis - mRNA vaccines contain polyethylene glycol (PEG), a primer that can cause life threatening reactions. “Seventy percent of people make antibodies to PEG and most do not know it, creating a concerning situation where many could have allergic, potentially deadly, reactions to a PEG-containing vaccine.”(10)
3) Lymphadenopathy - a swelling of lymph nodes
4) Infertility in women – the vaccines are expected to develop antibodies that attack spike proteins such as SARS-CoV-2. But these spike proteins are also essential in the formation of the human placenta. It’s not clear if the antibodies intended to attack SARS-CoV-2 would also attack the syncytin-homologous proteins essential for a successful pregnancy.(11)
5) HIV – subunits of HIV1 are in the vaccine which could lead to cancer, HIV and other autoimmune disorders.(12) Australia halted clinical trials because some participants had tested positive for HIV after taking the vaccine. Reports suggested these were “false positives” but the findings presented enough of a concern that Phase II/III trials were suspended.(13)
6) Altered genetic code – Figure 3 is a photo from the packaging of the AstraZeneca vaccine. Listed on the package is ChAdOx1-S [recombinant]. Recombinant describes the type of DNA technology used in the vaccine; it’s the “process of taking a gene from one organism and inserting it into the DNA of another”.(14) It is sometimes referred to as chimeric DNA because it’s a blending of two different species. Additionally, the mRNA technology uses a synthetic piece of DNA (synthetic means it’s not natural) which then gets replicated by mRNA and may be received by the genome, thereby altering the genetic code of a person.(15)
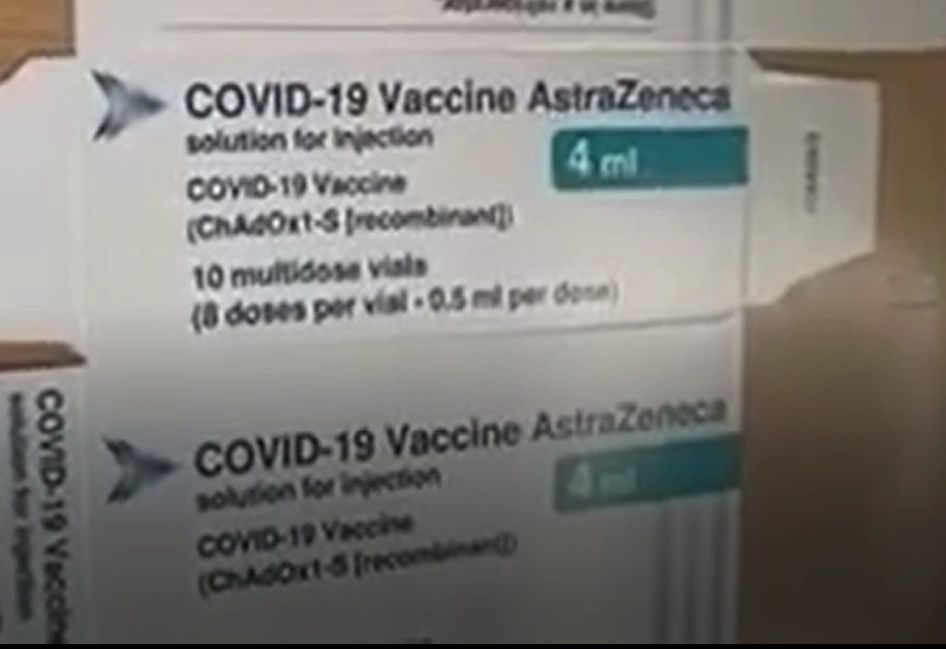
Figure 3. Packaging of AstraZeneca COVID-19 Vaccine. https://halturnerradioshow.com/index.php/en/news-page/world/we-ve-got-the-box-astra-zeneka-s-covid-19-vaccine-frankenstein-in-a-bottle
Figure 4 provides a list of additional risks from the SARS-CoV-2 vaccines as noted by the FDA Vaccines and Related Biological Product Advisory Committee. There have been

Figure 4 https://www.fda.gov/media/143557/download
participants in the vaccine trials who have developed Bell’s Palsy, transverse myelitis, anaphylaxis, and even death. Two days after the official release of the vaccine in the US, two healthcare workers from Alaska developed an anaphylactic response. One ended up in the hospital even though she had no prior history of allergies.(16) The Epoch Times reported that a hospital in Illinois suspended administering the vaccine because four health care workers developed tingling and increased heart rate. One of those workers needed additional medical treatment.(17) The FDA is now investigating allergic reactions in multiple states.(18)
**Update** - On 12/19/20, the CDC reports there have been 3,150 (2.8% of people receiving vaccine) “health impact events” that have left recipients of the vaccine unable to perform daily activities, unable to work, and require care from a doctor (see Figure 5). Don’t allow the term “health impact events” to soften the seriousness of these adverse events due to the vaccine. If a person is unable to perform daily activities this means they are unable to bathe themselves, feed themselves, brush their hair, get dressed etc. In psychological terms, this would mean severe mental illness. In medical terms, this would mean severe incapacitation, such as paralysis.
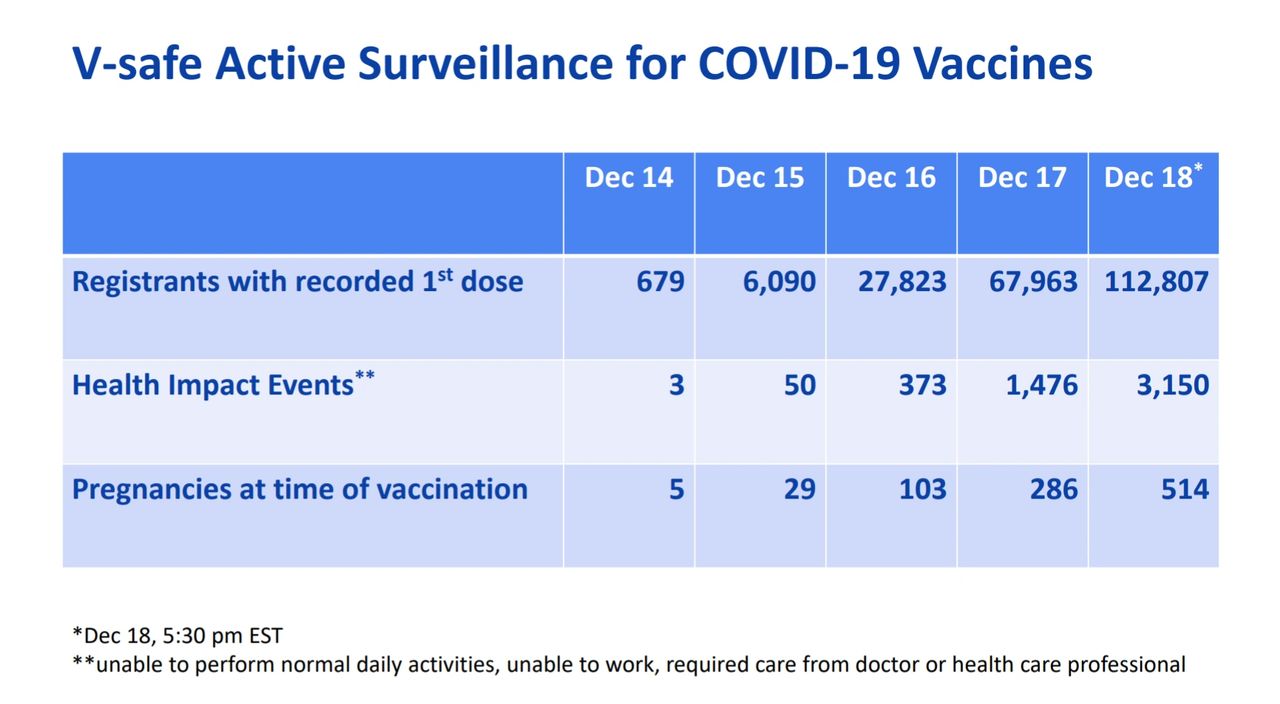
Figure 5. Health Impact Events per CDC. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/slides-12-19/05-COVID-CLARK.pdf
For a deeper dive in understanding the vaccines, please watch the below video.
Dr. Carrie Madej's Review of COVID-19 Vaccines
By Laura Sanger, Ph.D.
References
1. Haseltine, W. A. (September 23, 2020). Covid-19 Vaccine Protocols Reveal That Trials Are Designed to Succeed. Forbes. Retrieved from https://www.forbes.com/sites/williamhaseltine/2020/09/23/covid-19-vaccine-protocols-reveal-that-trials-are-designed-to-succeed/?sh=29c489ed5247
2. Ibid.
3. Boodman, E. (March 11, 2020). Researchers Rush to Test Coronovirus Vaccine in People Without Knowing How Well It Works in Animals. StatNews. Retrieved from https://www.statnews.com/2020/03/11/researchers-rush-to-start-moderna-coronavirus-vaccine-trial-without-usual-animal-testing/
4. Tseng, C. T. et al. (April 2012). Immunization with SARS Coronovirus Vaccines Leads to Pulmonary Immunopathology on Challenge with the SARS Virus. PLoS One 7, (4), pg. 1 – 13. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3335060/pdf/pone.0035421.pdf
5. Durder, T. (December 6. 2020). Ex-Pfizer Exec Demands EU Halt COVID-19 Vaccine Studies Over ‘Indefinite Fertility’ and Other Health Concerns. ZeroHedge. Retrieved from https://www.zerohedge.com/medical/ex-pfizer-exec-demands-eu-halt-covid-19-vaccine-studies-over-indefinite-infertility-and
6. Wodarg. W and Yeadon, M (December 1, 2020). Yeadon and Wodarg EMA Petition Pfizer Trial. Retrieved from https://www.scribd.com/document/487135032/Wodarg-Yeadon-EMA-Petition-Pfizer-Trial-FINAL-01DEC2020-en-Unsigned-With-Exhibits#fullscreen&from_embed
7. Ibid.
8. Divine Intel [YouTube] (Dec 16, 2020). Experimental Vaccine. Retrieved from https://www.youtube.com/watch?t=91&v=625-JLKY67o&feature=youtu.be
9. Amanda Forbes [YouTube] (Dec 17, 2020). Discussing Current Events with Dr. Carrie Madej. Retrieved from https://www.youtube.com/watch?v=xVWdxsMMvGY
10. Wodarg. W and Yeadon, M (December 1, 2020). Yeadon and Wodarg EMA Petition Pfizer Trial. Retrieved from https://www.scribd.com/document/487135032/Wodarg-Yeadon-EMA-Petition-Pfizer-Trial-FINAL-01DEC2020-en-Unsigned-With-Exhibits#fullscreen&from_embed
11. Ibid.
12. Amanda Forbes [YouTube] (Dec 17, 2020). Discussing Current Events with Dr. Carrie Madej. Retrieved from https://www.youtube.com/watch?v=xVWdxsMMvGY
13. Australia Halts Local Vaccine Development Due to False Positive for HIV (Dec 12, 2020). The Straits Times. Retrieved from https://www.straitstimes.com/asia/australianz/australia-halts-local-vaccine-development-due-to-false-positives-for-hiv
14. Recombitant. The Free Dictionary: Medical Dictionary. https://medical-dictionary.thefreedictionary.com/recombinant
15. Will New COVID Vaccine Make You Transhuman? (September 12, 2020). Mercola. Retrieved from https://articles.mercola.com/sites/articles/archive/2020/09/12/coronavirus-vaccine-transhumanism.aspx
16. Wieland, N., LaFreniere, S., Baker, M. and Thomas, K. (December 21, 2020). 2 Alaska Health Care Workers Got Emergency Treatment After Receiving Pfizer’s Vaccine. The New York Times. Retrieved from https://www.nytimes.com/2020/12/16/health/covid-pfizer-vaccine-allergic-reaction.html
17. Stieber, Z. (December 19, 2020). Hospital Halts COVID-19 Vaccinations After 4 Workers Have Adverse Reactions. The Epoch Times. Retrieved from https://www.theepochtimes.com/hospital-halts-covid-19-vaccinations-after-4-workers-have-adverse-reactions_3625380.html
18. Children’s Health Defense Team (December 21, 2020). FDA Investigates Allergic Reactions to Pfizer COVID Vaccine After More Healthcare Workers Hospitalized. The Defender. Retrieved from https://childrenshealthdefense.org/defender/fda-investigates-reactions-pfizer-covid-vaccine-healthcare-workers-hospitalized/?utm_source=salsa&eType=EmailBlastContent&eId=8c0edf71-f718-4f0d-ae2a-84905c9c8919